Collie Health
**NEW!**
UPDATE: 1/1/2024
We received 2 more test results. The results are Carrier for both so we will be working towards clearing this genetic marker.
Kodiac- RD Carrier
Sully - RD Carrier
UPDATE: 12/1/2023
Our first 2 tests came back today. The results are Carrier for both, so we will be continuing to test to make sure we breed away from this in our program.
Hunter - RD Carrier
Hattie - RD Carrier
_________________________________________________________________________________________________
UPDATE: 11/22/2023
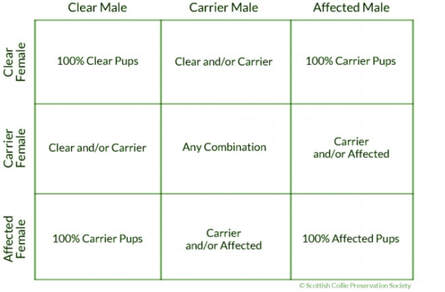
WE HAVE STARTED OUR NEW TESTING FOR RENAL DISPLAYSIA!! Our first 4 tests have been sent to the lab and results will be shared soon. We will be adding a new test to our program. A test in honor of "Key" and also in honor of "Darby" who were both lost to this terrible disease. Using this testing, we can use the results to go forward and breed those who carry 1 or 2 copies of the gene to those who are clear so we can work towards eliminating this in the dogs we breed. Renal dysplasia is not limited to puppies under the age of 2.
For many years the term “juvenile renal dysplasia” has been used to describe this form of inherited kidney disease. The mislabeling of renal dysplasia as juvenile condition has resulted in confusion about the disease, and consequently many breeders have assumed that the problem does not exist in their kennel. The age of onset is highly variable, and kidney failure from this birth defect can occur at any age, even in dogs that are up to 10 years of age or beyond. In fact more adults over the age of 5 will die from RD than puppies. Ultimately, this disease will shorten the life of many adults.
It is important to us to be the change we want to see taking this necessary step to a better future.
We received 2 more test results. The results are Carrier for both so we will be working towards clearing this genetic marker.
Kodiac- RD Carrier
Sully - RD Carrier
UPDATE: 12/1/2023
Our first 2 tests came back today. The results are Carrier for both, so we will be continuing to test to make sure we breed away from this in our program.
Hunter - RD Carrier
Hattie - RD Carrier
_________________________________________________________________________________________________
UPDATE: 11/22/2023
WE HAVE STARTED OUR NEW TESTING FOR RENAL DISPLAYSIA!! Our first 4 tests have been sent to the lab and results will be shared soon. We will be adding a new test to our program. A test in honor of "Key" and also in honor of "Darby" who were both lost to this terrible disease. Using this testing, we can use the results to go forward and breed those who carry 1 or 2 copies of the gene to those who are clear so we can work towards eliminating this in the dogs we breed. Renal dysplasia is not limited to puppies under the age of 2.
For many years the term “juvenile renal dysplasia” has been used to describe this form of inherited kidney disease. The mislabeling of renal dysplasia as juvenile condition has resulted in confusion about the disease, and consequently many breeders have assumed that the problem does not exist in their kennel. The age of onset is highly variable, and kidney failure from this birth defect can occur at any age, even in dogs that are up to 10 years of age or beyond. In fact more adults over the age of 5 will die from RD than puppies. Ultimately, this disease will shorten the life of many adults.
It is important to us to be the change we want to see taking this necessary step to a better future.
CEA (Collie Eye Anomaly)Collie Eye Anomaly (CEA), also known as choroidal hypoplasia (CH), is an inherited disease affecting several dog breeds including the Scottish collie. The choroid is the layer of tissue in the eye responsible for supplying blood and nutrients to the Retina. In dogs affected with CEA, the choroid does not develop properly and is therefore thinner than normal. The severity of the condition can vary from dog to dog. In mild cases, affected dogs may only show signs of collie eye anomaly on eye exam between about 5 and 12 weeks of age, just prior to normal, age-related pigmentation of the retina which often masks the characteristic, disease-related changes. After this time period, mildly affected dogs may be impossible to distinguish from normal dogs on eye exam (a phenomenon often referred to as “going normal”) and may not display obvious vision deficits. In more severely affected dogs, clinical signs include malformations of the eye and/or optic nerve (colobomas), retinal detachment, intraocular bleeding, and subsequent blindness. Both mild and severe forms of CEA are associated with the same NHEJ1gene Mutation. Therefore, predicting the potential severity of the disease in an affected puppy is difficult as mildly affected parents may produce offspring that are severely affected.
Breed-Specific Information for the Scottish Collie The Mutation of the NHEJ1 gene associated with collie eye anomaly has been identified in the Scottish collie, although its overall frequency in this breed is unknown. CN (Cyclic hematopoiesis)Cyclic neutropenia is an inherited disease affecting Scottish collies. Affected dogs undergo an oscillating cycle in the quantity of a type of white blood cells called neutrophils, important for controlling and preventing bacterial and fungal infections. Affected dogs have Neutrophil counts oscillating between normal quantities to almost zero neutrophils on an approximate 2 week frequency. Affected puppies often die within a few days of birth or are stunted in growth. Affected dogs have a gray coat color and are vulnerable to infections during periods of low neutrophil counts. Symptoms associated with this condition are normally seen during or immediately after a period of low neutrophil counts. Symptoms include fever, diarrhea, inflamed lymph nodes, gingivitis, lameness and mild bleeding episodes. Even with medical care, most dogs die before 2 years of age due to liver or kidney failure. VWD-II (Von Willebrand Disease)Von Willebrand disease type II (VWDII) is an inherited bleeding disorder affecting dogs. Dogs affected with VWDII have decreased levels and abnormal function of von Willebrand coagulation factor (vWf), which is an essential protein needed for normal blood clotting.
Affected dogs generally have moderate to severe signs of a bleeding disorder. Affected dogs may bruise easily, have frequent nosebleeds, bleed from the mouth when juvenile teeth are lost and experience prolonged bleeding after surgery or trauma. The bleeding may be severe enough to cause death. Due to variable severity of the disorder, affected dogs may not be identified until a surgery is performed or trauma occurs at which time excessive bleeding is noted. Veterinarians performing surgery on known affected dogs should have ready access to blood banked for transfusions. Dogs can have a normal lifespan with this condition although they are susceptible to life-threatening bleeding with an accidental injury or any surgical procedure. |
PRA (Progressive Retinal Atrophy)Progressive retinal Atrophy, Rod-cone dysplasia 2 is an early-onset, inherited eye disease affecting dogs. Progressive retinal atrophy, rod-cone dysplasia 2 occurs as a result of degeneration of both rod and cone type Photoreceptor Cells of the Retina, which are important for vision in dim and bright light, respectively. Affected dogs have abnormal thinning and degeneration of the retina beginning around 16 days of age. The rod type cells are affected first and affected dogs will initially have vision deficits in dim light (night blindness) and loss of peripheral vision noticeable at about 6 weeks of age. Other signs of progressive retinal atrophy involve changes in reflectivity and appearance of a structure behind the retina called the Tapetum that can be observed at 3.5-4 months of age on a veterinary eye exam. As the disease progresses, cone photoreceptor cells also degenerate resulting in complete blindness by 6 to 8 months of age. HUU (Hyperuricosuria)Hyperuricosuria is an inherited condition of the urinary system affecting several breeds of dog. The SLC2A9 gene codes for a protein that allows the kidneys to transport uric acid from the urine. Dogs with mutations in both copies of the SLC2A9 gene are predisposed to have elevated levels of uric acid in the urine, hence the name hyperuricosuria. Uric acid can form crystals and/or stones (uroliths) in the urinary tract. Dogs with hyperuricosuria most commonly present with symptoms of recurrent urinary tract inflammation, which include frequent urination, blood in the urine, and straining to urinate. They may also have loss of appetite, lethargy, weakness, vomiting and pain. Urinary stones in the bladder can cause urinary tract infections or more seriously, blockage of the Urethra. Both male and female dogs can be affected, but obstruction of urine flow is more common in males due to differences in anatomy. Although an x-ray can be used to exclude other types of stones, urate stones cannot typically be seen using x-rays and must be evaluated by ultrasound. Not all dogs with mutations in both copies of the SLC2A9 gene will have symptoms of disease, though they will have increased uric acid excretion in the urine. DM (Degenerative Myelopathy)Degenerative Myelopathy caused by Mutation of the SOD1 gene is an inherited neurologic disorder of dogs. This mutation is found in many breeds of dog, including the Scottish collie. While it is not clear for some of the other breeds, collies are known to develop degenerative myelopathy associated with this mutation. The variable presentation between breeds suggests that there are environmental or other genetic factors responsible for modifying disease expression. The average age of onset for dogs with degenerative myelopathy is approximately nine years of age. The disease affects the White Matter tissue of the spinal cord and is considered the canine equivalent to amyotrophic lateral sclerosis (Lou Gehrig’s disease) found in humans. Affected dogs usually present in adulthood with gradual muscle Atrophy and loss of coordination typically beginning in the hind limbs due to degeneration of the nerves. The condition is not typically painful for the dog, but will progress until the dog is no longer able to walk. The gait of dogs affected with degenerative myelopathy can be difficult to distinguish from the gait of dogs with hip dysplasia, arthritis of other joints of the hind limbs, or intervertebral disc disease. Late in the progression of disease, dogs may lose fecal and urinary continence and the forelimbs may be affected. Affected dogs may fully lose the ability to walk 6 months to 2 years after the onset of symptoms. Affected medium to large breed dogs, such as the rough collie, can be difficult to manage and owners often elect euthanasia when their dog can no longer support weight in the hind limbs. Recurrent Inflammatory Pulmonary DiseaseRecurrent inflammatory pulmonary disease is an inherited disease affecting dogs. Affected dogs present within the first week of life with signs of respiratory disease including coughing, nasal discharge, fever, vomiting, and shallow, noisy breathing. Dogs respond to treatment, but relapse quickly after treatment has ended. Some dogs may be maintained with medical therapy for years while continuing to have signs of respiratory disease throughout their life.
|
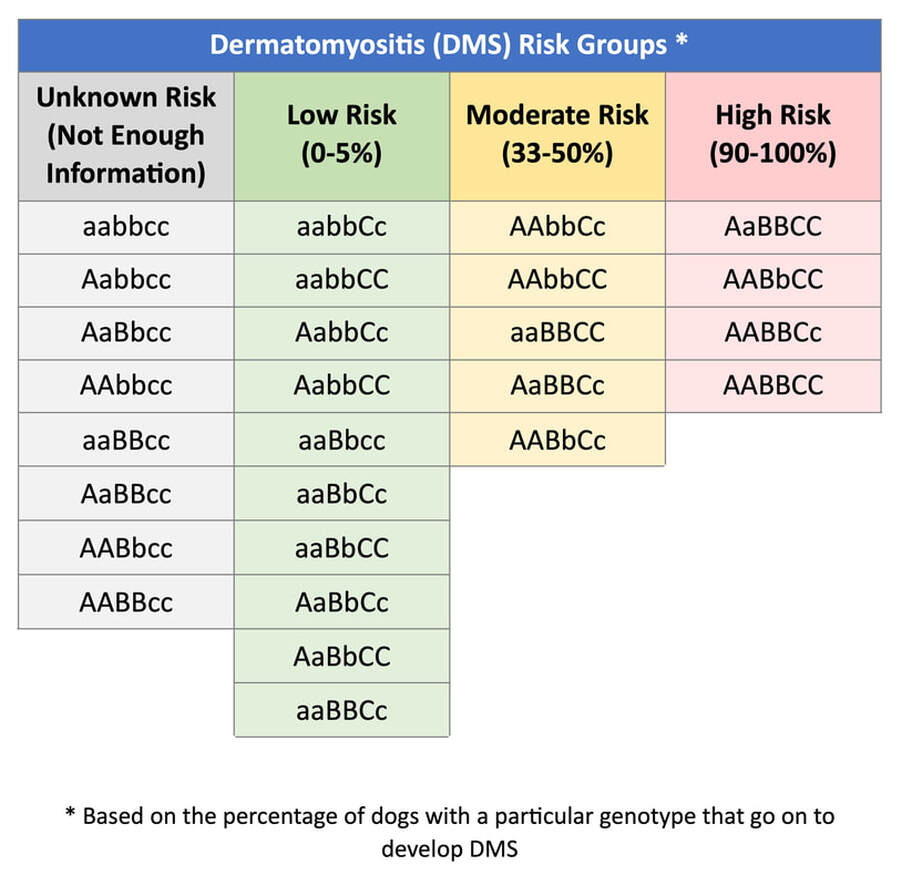
DMS (Dermatomyositis)
Dermatomyositis (DMS) is an immune mediated, inflammatory disease affecting the skin and muscles of Collies and Shetland Sheepdogs. Skin lesions can present before 6 months of age and typically precede muscle lesions. However, some dogs develop skin lesions later into adulthood. These wounds begin as small swellings that become pustules, ulcers, and crusty hairless regions. Affected areas include the muzzle, footpads, around the eyes and lips, ears, and any bony prominences like the points of the elbow or the tops of the paws. Unless secondarily infected, lesions are not painful or itchy. Typically, the disease peaks around 1 year of age, and in some cases the dog may improve as it continues to age. Some dogs with DMS may develop inflammation of the muscles (myositis) without showing skin lesions. Over time, muscles may show signs of Atrophy, especially the muscles needed for chewing. Affected dogs may have difficulty eating and drinking from a bowl and may walk with a stiff-legged gait. More commonly, skin lesions occur months before myositis develops. When present, myositis is consistent with overall disease severity, with mildly affected dogs presenting no signs of muscle issues. Dogs with severe myositis can exhibit stunted growth, widespread muscle atrophy, and aspiration pneumonia from swallowing difficulties. Treatment is focused on management of the presenting skin lesions along with regulating the immune system to reduce the presentation. Although most dogs will respond to treatment, relapses are common. Severe skin lesions may result in scarring. Long term complications are possible for dogs with chronic DMS lesions.
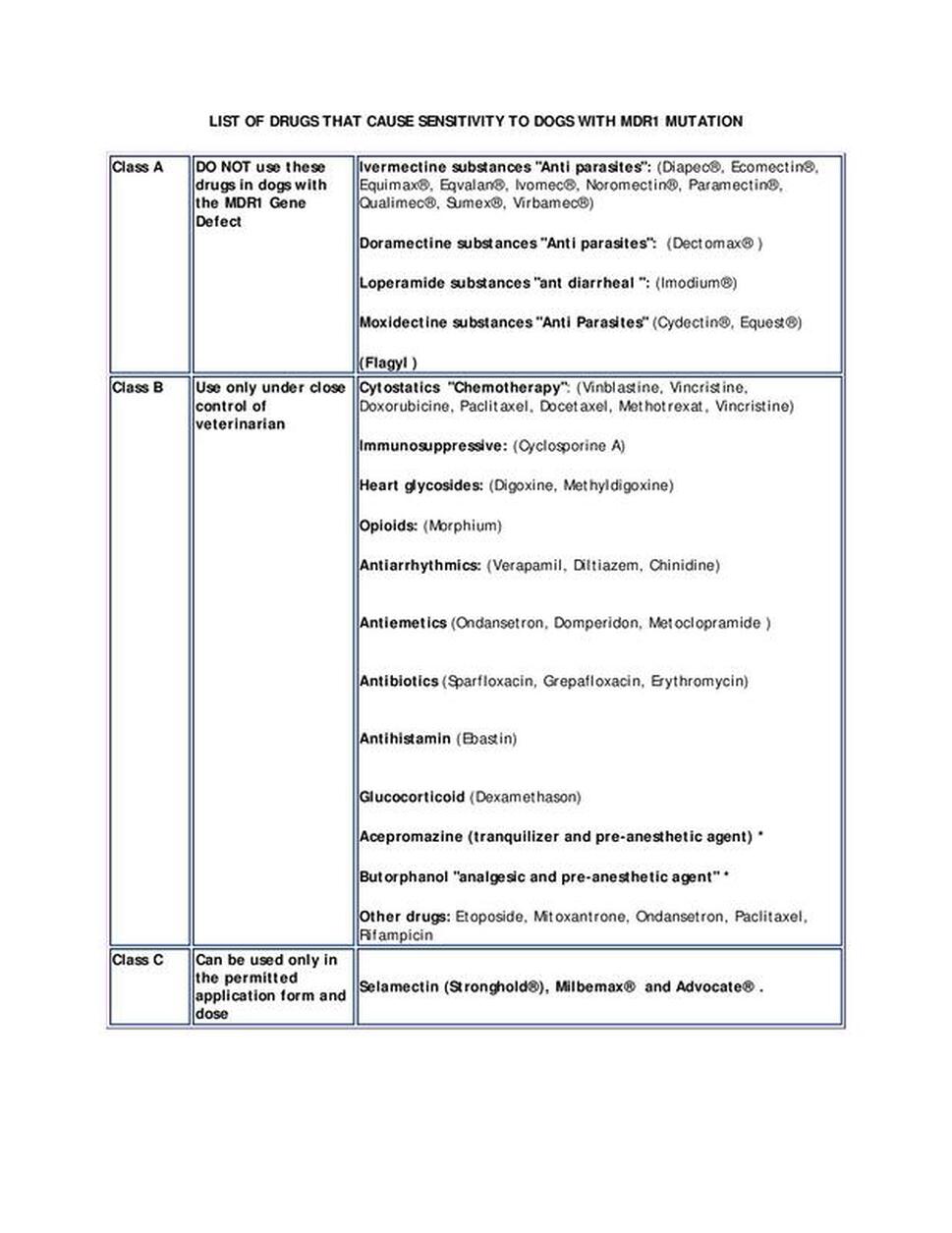
MDR1 (Multi-drug Resistance 1)
Multidrug resistance 1, also called MDR1, is an inherited condition affecting several breeds of dogs, especially herding dogs such as the Scottish collie. The Mutation in the ABCB1 gene associated with MDR1 causes dysfunction of P-glycoprotein, which is responsible for removing certain drugs and toxins from the body. Clinical signs are most commonly associated with distribution of the drug in the central nervous system. MDR1 is inherited in an autosomal incomplete dominant manner in dogs meaning that dogs only need to inherit one copy of the mutated gene to be at an increased risk of developing adverse reactions to certain medications. Though adverse reactions to certain drugs are most commonly seen in dogs having two copies of the mutated gene, Carrier dogs can also experience drug sensitivities and dosages need to be adjusted accordingly. Thus, dogs that have one or two mutant copies of the gene are considered at-risk for adverse drug reactions. If an at-risk dog is treated with one of several common drugs (see below*), they are at risk of developing neurologic symptoms that could range from tremors, excess salivation, anorexia, and blindness to coma and even death. Because of the defective ability to metabolize specific drugs, these drugs can be lethal even at low doses. The MDR1 mutation does not cause adverse effects in dogs unless the dog is exposed to these drugs. Therefore, veterinarians should be notified when a dog is at risk for multidrug resistance 1 prior to administration of any medications.
Credits for ALL Health information
Pawprint Genetics at https://www.pawprintgenetics.com
Credit for MDR1 list of drugs
Washington State University at https://vcpl.vetmed.wsu.edu/
Pawprint Genetics at https://www.pawprintgenetics.com
Credit for MDR1 list of drugs
Washington State University at https://vcpl.vetmed.wsu.edu/
"A healthy outside starts from the inside".